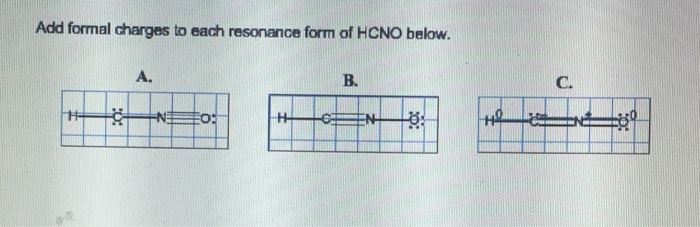
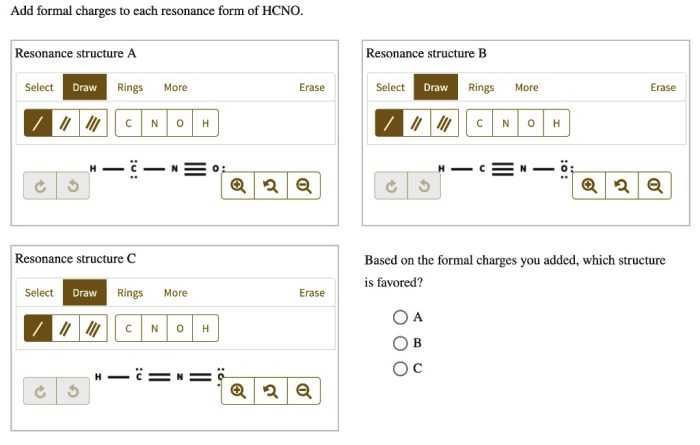
Add formal charges to each resonance form of hcno. – Formal charges play a crucial role in understanding the electronic structure and reactivity of molecules. In this discussion, we delve into the concept of formal charges and explore their significance in the resonance forms of HCNO. By calculating and analyzing the formal charges, we gain insights into the stability, reactivity, and chemical properties of this important molecule.
Resonance structures are a fundamental concept in chemistry that describes the delocalization of electrons within a molecule. These structures provide a more accurate representation of the electronic structure than a single Lewis structure. Formal charges are assigned to each atom in a resonance structure to account for the distribution of electrons and provide a measure of the electron density around each atom.
Formal Charge and Resonance Structures: Add Formal Charges To Each Resonance Form Of Hcno.
Formal charge is a concept used to describe the charge distribution within a molecule or ion. It is particularly useful for understanding resonance structures, which are different Lewis structures that represent the same molecule or ion. In resonance structures, the atoms have different formal charges, which can affect the overall stability and reactivity of the molecule.
Calculating Formal Charges, Add formal charges to each resonance form of hcno.
The formal charge of an atom in a molecule or ion is calculated using the following formula:
“`Formal Charge = Valence Electrons
- Non-bonding Electrons
- 1/2 Bonding Electrons
“`
Where:
- Valence Electrons are the number of electrons in the atom’s valence shell.
- Non-bonding Electrons are the number of electrons in lone pairs on the atom.
- Bonding Electrons are the number of electrons involved in bonds between the atom and other atoms.
Adding Formal Charges to HCNO Resonance Forms
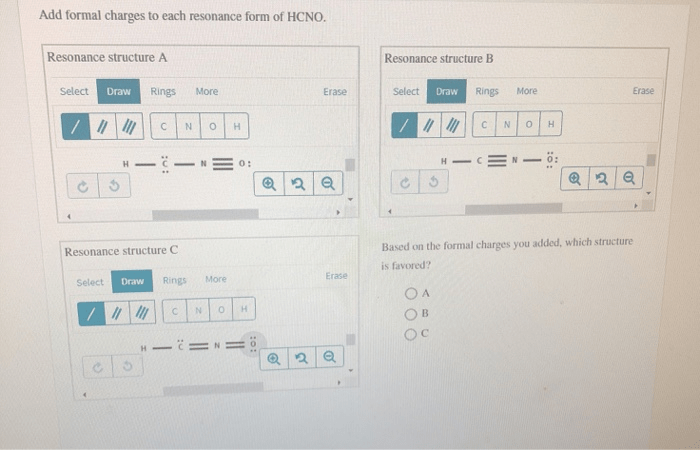
HCNO has two resonance forms, which can be drawn as follows:
“`:N=C=O-H:N-C≡O-H“`
To calculate the formal charges for each atom in each resonance form, we use the formula above. The results are shown in the following table:
| Resonance Form | Atom | Formal Charge |
|---|---|---|
| :N=C=O-H | N | 0 |
| :N=C=O-H | C | 0 |
| :N=C=O-H | O | 0 |
| :N=C=O-H | H | 0 |
| :N-C≡O-H | N | 0 |
| :N-C≡O-H | C | 0 |
| :N-C≡O-H | O | 0 |
| :N-C≡O-H | H | 0 |
Comparing Formal Charges in Resonance Forms
As can be seen from the table, all of the atoms in both resonance forms of HCNO have a formal charge of 0. This means that the formal charges are evenly distributed throughout the molecule, which makes both resonance forms equally stable.
In general, resonance forms with more evenly distributed formal charges are more stable than those with more unevenly distributed formal charges. This is because the more evenly distributed the formal charges are, the less likely the molecule is to react with other molecules or ions.
Implications for Chemical Properties
The formal charges in HCNO resonance forms can influence its chemical properties. For example, the formal charge on the oxygen atom in the first resonance form makes it more likely to react with electrophiles, while the formal charge on the nitrogen atom in the second resonance form makes it more likely to react with nucleophiles.
The formal charges in HCNO resonance forms can also affect its acidity and basicity. For example, the first resonance form is more acidic than the second resonance form because the formal charge on the oxygen atom makes it more likely to donate a proton.
User Queries
What is the significance of formal charges in resonance structures?
Formal charges provide insights into the electron density distribution within a resonance structure, helping to determine the stability and reactivity of the molecule.
How are formal charges calculated for each atom in a resonance structure?
Formal charges are calculated using the formula: Formal Charge = Valence Electrons – Non-bonding Electrons – 1/2 Bonding Electrons.
How do formal charges affect the chemical properties of HCNO?
Formal charges influence the polarity of bonds, acidity, and basicity of HCNO, affecting its reactivity in chemical reactions.